Abstract
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection results in poor outcome in patients with hematologic malignancies. Moreover, the efficacy of anti-SARS-CoV-2 mRNA vaccines appears lower in immunocompromised patients, including recipients of allogeneic stem cell transplantation (Allo-HSCT). In this population, data are scarce regarding factors predicting the response to mRNA vaccines.
Methods
This retrospective study aimed to decipher which factors, including immune status at time of vaccine and recipient/donor blood groups, might influence the antibody response after two injections (V1 and V2) of BNT162b2 (Pfizer-BioNTech) vaccine in a cohort of allografted patients with no previous symptomatic nor asymptomatic COVID-19 infection. Possible previous asymptomatic COVID-19 infection was investigated in pre-V1 samples by testing for anti-nucleocapsid (N) antibodies (anti-SARS-CoV-2 immunoassay, Roche Elecsys®, Rotkreuz, Switzerland). Antibody response to the SARS-CoV-2 spike protein receptor-binding domain was tested post-V2 (Roche Elecsys®). As recommended by the manufacturer, titers ≥0.8 U/mL were considered positive, the highest value being >250 U/mL.
Blood samples were also collected before V1 and at distance from V2 to evaluate, by flow cytometry, total lymphocyte (Ly) counts and quantitative Ly subsets (CD3, CD4 and CD8 T cells, B and NK cells).
Statistical analyses were performed on R (version 4.0.3). Patient characteristics were compared by using the Χ² test for discrete variables and the Wilcoxon test for continuous variables. Generalized linear models were used to conduct multivariate analyses.
Results
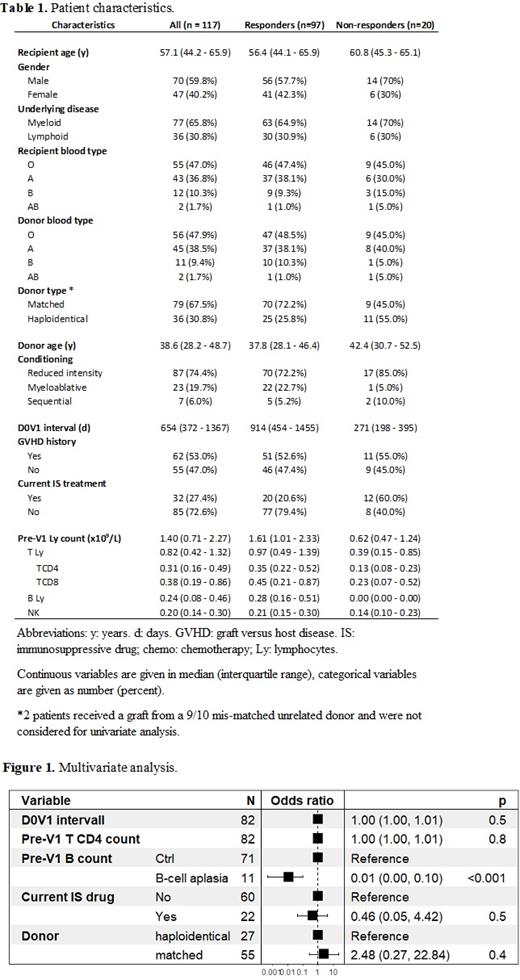
Samples were available from 117 Allo-HSCT patients who had been vaccinated between January 20 and April 17, 2021. Patient characteristics are provided in Table 1. The average interval from Allo-HSCT day 0 (D0) to V1 (D0-V1) was 654 (IQR: 372-1367) days (d). S-antibody response rate post-V2 was 82.9% for the entire cohort. Non-humoral responders (NHR) post-V2 (n = 20) had a lower D0-V1 interval (median 271 vs 914 d, p <10 -5) and a lower pre-V1 median total Ly count (0.62 vs 1.61x10 9/L, p < 10 -4). Lymphocyte subsets possibly predictive of antibody response were then investigated. NHR were associated with lower median CD3 (0.39 vs 0.97 x10 9/L, p = 0.01), CD4 (0.13 vs 0.35 x10 9/L, p=<10 -3), and B-cell (0.00 vs 0.28 G/L, p <10 -6) counts. NK and T CD8 counts were not statistically different between NHR and HR (respectively p=0.14 and p=0.06). No influence either was observed when considering the age of donors (p=0.39) or recipients (p=0.55), underlying disease (p=1), Allo-HSCT conditioning (p=0.11), blood groups (donor, p=0.55; receiver, p=0.39) or a previous history of graft versus host disease (GVHD; 83.1 vs 83.6%, p=1). Conversely, ongoing immunosuppressive (IS)/chemotherapy treatment and a haploidentical source of graft were associated with lower responses to vaccination (respectively 62.5 vs 90.5%, p<10 -3, and 69.4 vs 88.6% for patients with matched donors, p=0.02). In multivariate analysis (Fig.1) also including D0-V1 interval, donor source, current IS/chemotherapy treatment and TCD4 Ly count, only B cell aplasia remained statistically associated with lack of antibody response after two vaccine injections (OR 0.01, 95%CI [0.00 - 0.10], p <10 -3).
The possible modification in terms of lymphocyte counts between pre-V1 and post-V2 times has been also investigated showing that only CD4 lymphocytes counts improved significantly (0.31 vs 0.34 x10 9/L, p=0.01) between this interval.
Conclusion
B cell aplasia appears as a major predictor of anti SARS-CoV-2 mRNA vaccine failure after Allo-HSCT. It may be suggested from this result that a close immune monitoring should be proposed after allotransplant to propose the vaccine at the most appropriate time, meaning after of B cell detection, regardless of the delay from Allo-SCT or the presence of an IS/chemotherapy treatment. The possibility for these patients to have mounted a cellular response should also be considered, which was not investigated here.
Moreau: Celgene BMS: Honoraria; Sanofi: Honoraria; Abbvie: Honoraria; Oncopeptides: Honoraria; Amgen: Honoraria; Janssen: Honoraria.